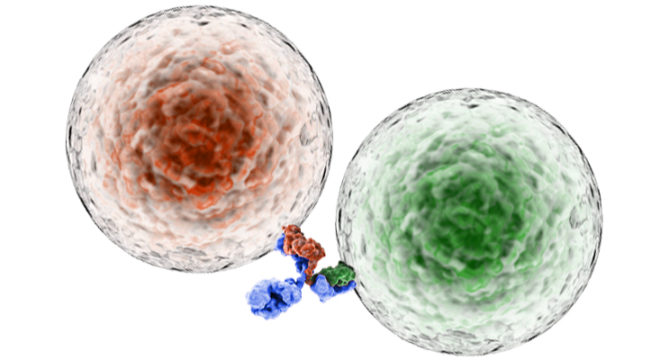
What is a bi-specific antibody?
Bi-specific antibodies are a class of engineered antibodies developed for various oncology indications and autoimmune diseases. Typically, the antigen recognition domains on the tips of the F(ab)2 fragment of a standard antibody are identical and bind the same antigen. In contrast, bi-specific antibodies have different antigen recognition domains on each of those tips and each tip will thus bind a different antigen.
With advances in genetic and protein engineering and recombinant protein expression, different types of bi-specific antibodies can be produced. While there are a variety of bi-specific antibodies that are found in at least 50 different formats, they fit into two common types. One is in the form of a standard antibody with a F(ab)2 and Fc region, while the other is in a form lacking the Fc region. The standard antibody form retains Fc function to mediate antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) due to the presence of the Fc region. These are mechanisms in which the Fc portion of the antibody drives to eliminate targeted/diseased cells independent of the antigens the antibodies bind (please see our previous blogs on ADCC and CDC for more information). In contrast, the other form of bi-specific antibodies that lack the Fc region are unable to facilitate these processes.
How do bi-specific antibodies work?
The F(ab)2 region of the bi-specific antibody can act by two other mechanisms of action separate from the effector functions of the Fc region. The first mechanism, which the majority of bi-specific antibodies are designed for, is to serve as a bridge to bring two different cell types into proximity, usually the recruitment of immune cells to cancer cells. By bringing these cells together, the immune cell will become activated and destroy the cancer cell. In this case, one arm of the antibody will bind an antigen expressed on the immune cell, such as CD3 for T cells or CD56 for NK cells. The other arm will bind an antigen expressed on the diseased cell, such as CD19 or HER2, to bring it into the proximity of the immune cell. Once the cells are close enough, activation signals are triggered on the immune cell, leading to lysis of the diseased cell. In fact, an immune synapse, a molecular event required for activation usually triggered by immune receptor engagement to their cognate ligands, is formed with bi-specific antibody activation of immune cells. Two approved bi-specific antibodies that use this mechanism of action are: 1) blinatumomab, where one arm recognizes CD3 and the other binds CD19, for CD19 expressing diseases, and 2) catumaxomab, where CD3 and EPCAM are the antigens, for malignant ascites.
Bi-specific antibodies that are predicated on this concept of bringing objects together can also be applied to soluble molecules. Emicizumab, a bi-specific antibody currently being developed by Chugai, binds both activated coagulation factor IX and inactivated factor X to promote activation of the latter. In this case, factor IX is performing the function of factor VIII, which is missing in hemophilia A patients, to help restore the normal blood clotting process.
The second mechanism of action by which bi-specific antibodies work is through interference of receptor signaling, which is similar to the way mono-specific therapeutic antibodies, such as Herceptin, operate. For example, in a disease setting, the target of Herceptin, HER2, is overexpressed and dimerizes without ligands to initiate a signaling cascade that promotes survival and proliferation of cancer cells. Herceptin blocks dimerization of this receptor and, thus, the subsequent activation of downstream signaling events that drive cancer growth. However, cancer cells can activate other survival pathways to compensate for compromised signaling events. With a bi-specific antibody, it interferes with both signaling pathways by binding the receptors to prevent initiation of their respective downstream signaling events. Alternatively, the arms of the bi-specific antibody can bind the ligands for the receptors to prevent the ligand’s occupation of the receptor, thereby blocking the initiation of signaling events driven by the receptors. Examples of bi-specific antibodies that use this mechanism of action include Roche’s vanucizumab (RG7221), which binds soluble factors Angiopoietin-2 and VEGF, and Genentech’s duligotuzumab (RG7597), which bind the HER1 and HER3 receptors. Therefore, these bi-specific antibodies work by interfering with the initiation of signaling events that drive cancer cell growth.
Join us next time for “Antibodies with a split personality…(Part 2)” when we discuss the breakthroughs that advanced the field of bi-specific antibodies and the aspects of technical development they address. Then in part 3, our final blog in the series, we will discuss assays designed for preclinical characterization of bi-specific antibodies, specifically those relevant for IND enabling studies.