Cryopreserved Samples: To use or not to use…
Why use cryopreserved samples?
Many of us envy our colleagues that use cell lines for their experiments because the cells are so readily available and abundant. The cells keep growing with little maintenance and there is no restriction on the number of cells required. In addition, the use of cell lines provides flexibility as to when the experiments can be done, whether it’s tomorrow or a year from now. The cell lines are simply frozen and then thawed when their services are required.
However, there are many situations where the cells, particularly primary samples, are in limiting numbers or simply rare, such as cancer samples. Moreover, processing these samples can be quite time consuming and difficult. Assuming the researcher has the time, reagents, and equipment to process the samples, the experiments still have to be setup and performed afterwards. All this would make for one very long day. Thus, having the option to use cryopreserved cells provides flexibility and, if available, multiple samples can be combined to provide enough cells to perform the experiment.
Experimentally, cryopreserved samples can be used for tracking the progression of a particular disease or response in a particular individual. In these longitudinal studies, samples are cryopreserved at the desired timepoints and subsequently analyzed once they are all collected to prevent day-to-day variability. An example would be a clinical trial or animal pharmacodynamic study.
In some instances, samples are shipped from various locations to a central site where the experiments are performed in order to prevent inter-site experimental variability. Because the distance to the central site can be quite far, the best way to maintain good viability of the cells during the shipment is by cryopreserving them.
Finally, using cryopreserved samples is an alternative for researchers who do not have the capabilities or facilities to obtain primary human or animal samples and do not want to use cell lines. Use of primary samples is more “physiological” and the results may contain fewer artifacts compared to cell lines, where its mutation(s) may contribute to the findings.
Together, using cryopreserved samples provide technical as well as experimental flexibility and consistency.
Limitations of using cryopreserved samples
However, the question is: do cryopreserved samples resemble fresh samples in terms of population characteristics, cell surface protein level, and function?
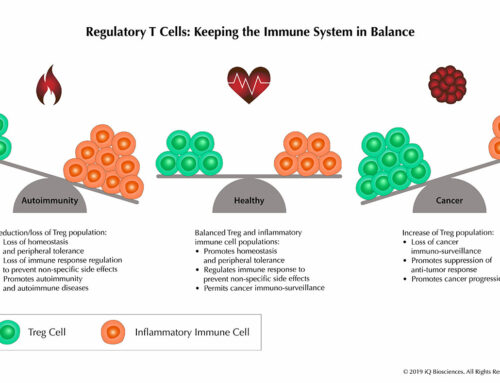
Early studies demonstrated that cryopreservation had an effect on PBMC subsets and the expression level of cell surface proteins. Freezing altered the ratio of plasmacytoid to conventional dendritic subsets, while in B cells, there was significant loss of the CD19+ subset1. Another subset of cells that was reduced in frequency after cryopreservation was T regulatory (CD4+ Foxp3+) cells1. In addition, cryopreservation had an effect on the expression level of cell surface molecules since freezing led to loss of CD62L and CCR5 expression1, 2.
Functionally, cryopreservation alters the secretion of some cytokines, chemokines, and even expression of transcription factors. Some examples include IFN-g, IL-10, TGF-β, and Foxp31. Thus, the early consensus was that using cryopreserved samples can result in inconsistent and confusing data.
However, more recent studies suggest that once an optimized standard operating protocol for cryopreservation and thawing is established, frozen samples can look and behave similarly to fresh ones. One study using optimized cryopreservation and thawing conditions found similar percentages of CD4 and CD8 cells between fresh and 15 month frozen samples, as well as in certain T cell subpopulations2. However, the same study did observe that CD45RO and CD62L expression differed between fresh and cryopreserved samples2, suggesting that the freezing procedure may affect certain cell surface proteins.
In the same study, the authors also examined functional response to tetanus, candida, and pokeweed in fresh and frozen PBMCs by lymphocyte proliferation assays2. Again, following their validated freezing and thawing protocol, the study found that fresh and frozen PBMCs proliferated similarly.
Another study demonstrated that an established optimized protocol for cryopreservation and thawing shared by multiple sites resulted in less variability and increased sensitivity when assaying for antigen specific T cells by tetramer staining or for function by ELISPOT3.
Together, these studies suggest that although cryopreservation may change the phenotype of the sample, optimizing cryopreservation and thawing procedures can minimize these alterations.
Considerations for using cryopreserving cells
Cryopreserving primary and cancer samples require more care and consideration relative to cell lines because it is imperative to maintain a high level of viability and prevent changes in phenotypic characteristics as described above. Thus, details such as type of serum, cryoprotective agent, medium, the freezing process, and the thawing process are important factors to consider.
Recent studies have suggested that freezing human PBMC samples in 10% DMSO diluted in FBS or in 12.5% human serum albumin (HSA)/87.5% RPMI resulted in the best cell viability1,4,5. However, because HSA sources and its preparation differ, quality and performance may vary. As an alternative, one study demonstrated that DMSO diluted in Invitrogen AIM-V medium, which contains HSA, results in better recovery of cells1,6.
One factor that seems to be agreed upon, from academic institutions to industry, is to slowly decrease the temperature during freezing1. Although there is no consensus on the temperature of the freezing medium (4° or 25° C), there is consensus that the cells should be gradually decreased to -70° C. The cells are then transferred to liquid nitrogen 24-72 hours after that.
In addition, the most commonly recommended way of thawing cells is by transferring them to 37° C directly from liquid nitrogen and then quickly washing away the DMSO with pre-warmed media1. One study and some industry standard protocols recommend slowly adding the pre-warmed media to increase viability2.
Validation of cryopreserved samples
Using cryopreserved samples is becoming more common and accepted, but the user should validate that the cryopreserved samples are phenotypically similar to fresh ones. Validating requires that the user compare fresh against frozen samples after an optimized standard operating protocol (or test conditions) has been established for cryopreservation and thawing. Ideally, cryopreserved samples are thawed periodically to check for the phenotypes of interest. This includes examining the cell surface proteins of interest for population distribution and expression level. If functional assays are required, the researcher should ensure that the frozen sample is performing similarly to the fresh sample using the desired assay. Unfortunately, validating may take some time, but the results will be more reliable and interpretable.
If cryopreserved samples are obtained from outside sources such as iQ Biosciences, the user should be aware of the source of the sample and whether or not they have a standard operating protocol for cryopreservation and thawing. For instance, iQ Biosciences provides their standard operating protocols for cryopreservation7 and thawing8 of their cryopreserved samples on their website. Further, the researcher should ask the source how they validate their cryopreserved samples and for documentation of their findings.
Like stated above, the use of cryopreserved samples is becoming more prevalent and accepted. In addition, it is becoming more apparent that optimizing cryopreservation and thawing protocols will help consistency between fresh and frozen samples. Researchers should take care in validating their cryopreserved samples before relying on them and making critical experimental conclusions, but the use of frozen samples provides many technical and experimental advantages.
For technical advice or questions, please email us at info@iqbiosciences.com.
References:
1. Mallone, R. et al. Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T cell responses: position statement of the T-Cell Workshop Committee of the Immunology of Diabetes Society. Clinical and experimental immunology 163, 33-49 (2011).
2. Weinberg, A. et al. Optimization and limitations of use of cryopreserved peripheral blood mononuclear cells for functional and phenotypic T-cell characterization. Clinical and vaccine immunology : CVI 16, 1176-1186 (2009).
3. Britten, C.M. et al. The CIMT-monitoring panel: a two-step approach to harmonize the enumeration of antigen-specific CD8+ T lymphocytes by structural and functional assays. Cancer immunology, immunotherapy : CII 57, 289-302 (2008).
4. Bull, M. et al. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. Journal of immunological methods 322, 57-69 (2007).
5. Disis, M.L. et al. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. Journal of immunological methods 308, 13-18 (2006).
6. Martinuzzi, E. et al. Serum-free culture medium and IL-7 costimulation increase the sensitivity of ELISpot detection. Journal of immunological methods 333, 61-70 (2008).
7. Duramad, O, et al iQ SOP Cryopreservation of PBMCs V1
8. Duramad, O, et al iQ SOP Thawing of cryopreserved samples V1