Cytokine Storms a-brewing… (Part 2)
Considerations for CRAs?
This is Part 2 of our series, “Cytokine Storms a-brewing…”. Miss Part 1? Catch up here.

When are cytokine release assays (CRA) required?
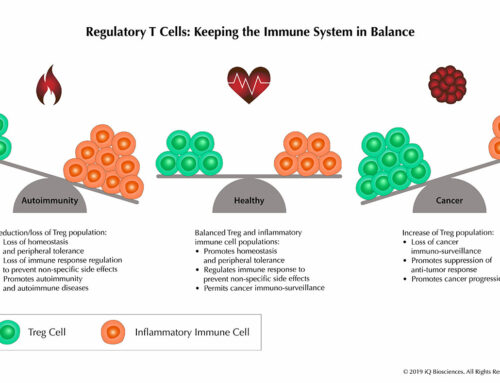
In the case of TGN1412, the biologic was an antibody. However, any product- whether a large biological molecule or small chemical- that has the potential to interact with the immune system should be tested. Furthermore, products targeting membrane-bound antigens could pose a higher risk for promoting cytokine release. Given that cancer immunotherapy is an increasingly popular field and many of its targets modulate the immune system, the products designed against those targets should no doubt be subjected to CRAs. According to “Guidance for Industry Immunogenicity Assessment for Therapeutic Protein Products”1, data from these in vitro studies should also be taken as an indication that the product has the potential to induce toxicities in the clinic, regardless of negative findings from preclinical animal studies.
An additional benefit besides identifying a potential hazard, CRAs can also be used to select the best therapeutic antibody candidate. In this case, companies can rank their potential candidates for the same target antigen based on its potential in vivo risk.
A typical startup biotech has a candidate target in mind and has developed a biologic to modulate that target. However, in many situations, the company is ill-equipped or doesn’t have the expertise to perform CRAs with their biologic. Contract research organizations (CROs), such as iQ Biosciences, have begun to perform CRAs for biotech companies because they have the expertise and knowledge to do them correctly and efficiently.
What are CRAs?
CRAs are assays designed to determine if human lymphocytes will make cytokines in response to test biologics, whether it’s a therapeutic antibody or small molecule. Although certain studies have indicated that IL-2, IFN-γ, and TNF-α are the best prognosticators of a potential cytokine storm2, 3, the best CRAs will examine a panel of cytokines that also include IL-6.
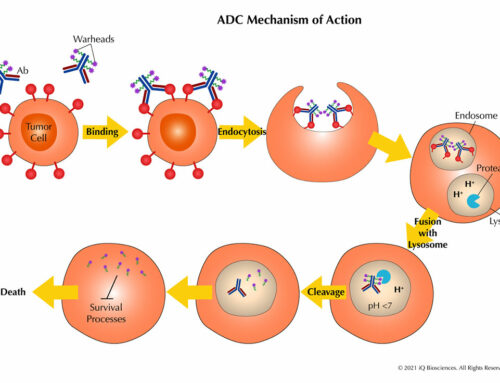
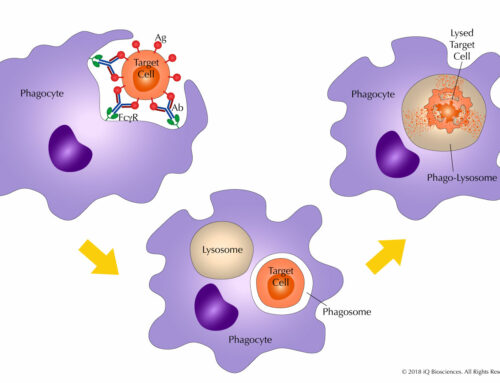
The assays are designed to culture human lymphocytes in the presence of the test biologic. The supernatant is then collected and tested by a variety of formats, which include simple cytokine ELISAs, LI-COR Human Cytokine Array, or Human Cytometric Bead Array which is the choice of iQ Biosciences (visit the iQ Biosciences CRA page for more information). Other methods use proprietary reagents and instruments, which may drive up cost.
Considerations for performing CRAs
As mentioned above, one of the reasons why the in vitro studies failed to predict the TGN1412-mediated cytokine storm was that the antibody was not “presented” in its most potent form. In studies after the TGN1412 clinical trial, it was determined that antibodies dry- or wet-coated onto plates are more potent than antibodies in solution4. Thus, many CRAs testing potential therapeutic antibodies will require coating the plates with the biologic.
Another finding from the post-trial studies was the cellular source of the cytokines. Most of the cytokines produced in response to TGN1412 came from CD4 effector memory cells3 but other cell types, such as NK cells, CD8 cells, and macrophages can also produce cytokines2. More importantly, a short culture time with PBMCs may not give the lymphocytes enough time to differentiate into effector cells (i.e. memory cells) that have the capacity to secrete cytokines. This scenario is misleading: not giving enough culture time for the lymphocytes to differentiate and respond to the biologic will lead to a false negative and a potential cytokine storm in vivo2. In addition, PBMCs only contain 2-5% lymphocytes and may therefore under-estimate the cytokine response2.
Assays to determine if lymphocytes proliferate in response to the biologic may also be performed with the longer culture time. Studies after the phase I trial of TGN1412 demonstrated that lymphocytes definitely proliferated in response to it, but only after 48-72 hours of culture time2.
With these considerations in mind, selecting a CRO with expertise in performing CRAs is paramount in light of the current trend in therapeutics that is aimed to modulate the immune system. The CRO must validate the CRAs with good positive and negative controls, but also know the details of how the therapeutic is presented. In addition, they should know which cytokines are most indicative of potential adverse events in vivo. However, they should also assay other cytokines to develop an overall picture of the cytokine profile as well as other methods to corroborate these findings.
References:
- Guidance for Industry Immunogenicity Assessment for Therapeutic Protein Products. FDA DRAFT GUIDANCE (Clinical/Medical, February 2013).
- Stebbings, R., Eastwood, D., Poole, S. & Thorpe, R. After TGN1412: recent developments in cytokine release assays. Journal of immunotoxicology 10, 75-82 (2013).
- Eastwood, D. et al. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. British journal of pharmacology 161, 512-526 (2010).
- Stebbings, R. et al. “Cytokine storm” in the phase I trial of monoclonal antibody TGN1412: better understanding the causes to improve preclinical testing of immunotherapeutics. J Immunol 179, 3325-3331 (2007).
Stay tuned for Part 2 of our series, “The Power of Complements…”! We’ll be discussing when complement-dependent cytotoxicity (CDC) assays are used and the key considerations for performing them.