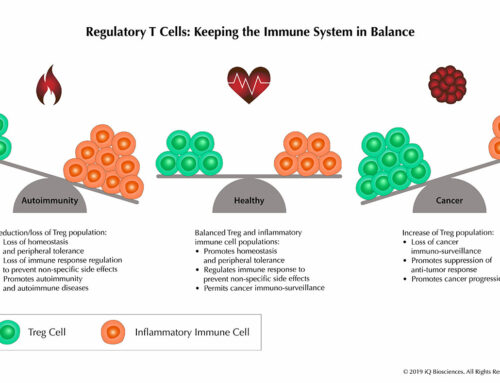
 The complement system of the innate immune system has been widely regarded as an early deterrent to infections and a means to clear pathogens by activating a group of proteins that leads to cell lysis, phagocytosis, and inflammation. In addition, the power of complement has been implicated as a mechanism by which therapeutic antibodies, such as Rituxan, promoted their anti-tumor effects. Along those lines, many biotechnology and pharmaceutical companies have tried to harness that power in targeted monoclonal antibody (mAb) cancer therapies. However, more recent findings have indicated that, like everything about the immune system, balance is key and dysregulation of complement can in fact promote tumorigenesis.
The complement system of the innate immune system has been widely regarded as an early deterrent to infections and a means to clear pathogens by activating a group of proteins that leads to cell lysis, phagocytosis, and inflammation. In addition, the power of complement has been implicated as a mechanism by which therapeutic antibodies, such as Rituxan, promoted their anti-tumor effects. Along those lines, many biotechnology and pharmaceutical companies have tried to harness that power in targeted monoclonal antibody (mAb) cancer therapies. However, more recent findings have indicated that, like everything about the immune system, balance is key and dysregulation of complement can in fact promote tumorigenesis.
In this blog, we will take a different approach from our previous ones that focused on certain topics and instead summarize a recent review from Reis et al (Reis, ES, Mastellos, DC, Ricklin, D, Mantovani, A, and Lambris, JD. Complement in cancer: untangling an intricate relationship. Nature Reviews Immunology, Volume 18(1), 5-18 (2018). doi: 10.1038/nri.2017.97) that discusses these new findings on the complement system and provide some understanding into the clinical aspects that target complement.
Complement and cancer therapy
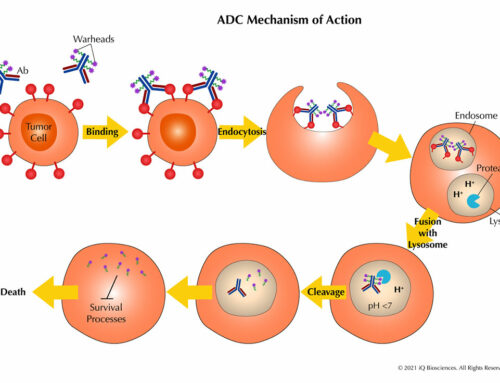
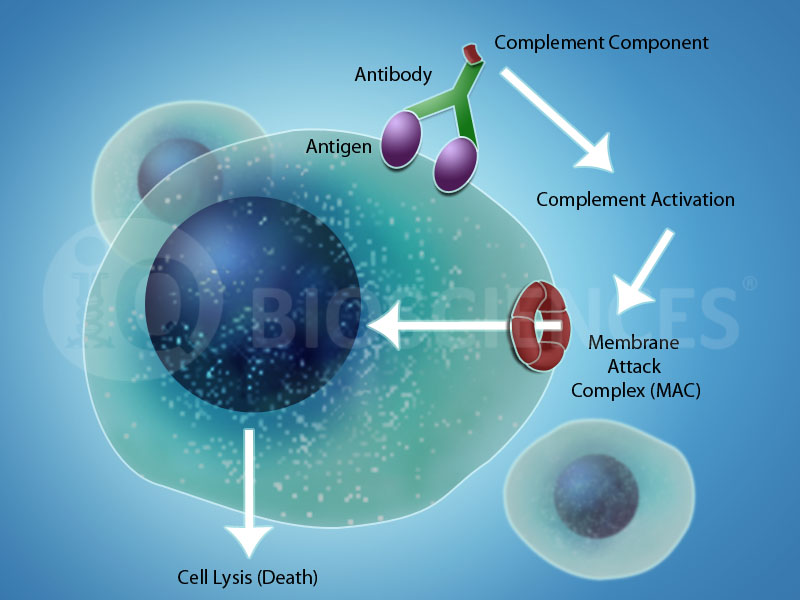
As alluded to earlier, mAb-based targeted cancer therapies attempt to harness the power of the complement system to initiate complement-dependent cytotoxicity (CDC) to promote its anti-tumor effects. There are three main pathways associated with CDC: 1) the classical pathway, 2) the alternative pathway, and 3) the Lectin pathway. Although initiation of the three pathways are distinct, there is considerable overlap between the complement cleavage cascade that all end in formation of the membrane attack complex (MAC), which functions to create openings in the plasma membrane of the target cell to promote death by osmotic lysis or opsonization.
The complement system itself consists of over 30 different components ranging from soluble factors to membrane-bound proteins, such as complement receptors and regulatory proteins that promote or inhibit complement activity and function. The initiators of the complement pathways are inactive until a trigger is received, whereupon these proteins are cleaved into their active forms to initiate a cascade of complement protein cleavages that culminates in MAC formation.
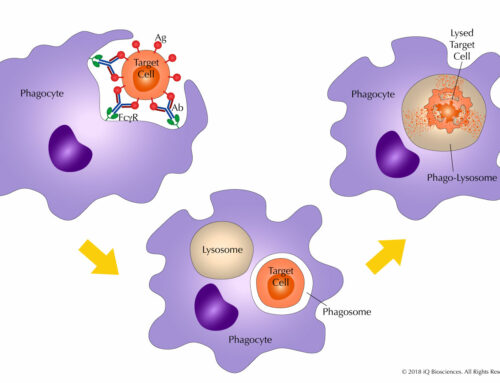
In the classical pathway by which most targeted mAbs therapies work, antibodies complexed with antigens or pathogen-associated molecular patterns (PAMPs), along with the complement protein C1q, drive this pathway. Here, C1q is recruited to the target cell/pathogen by the antibody/antigen (or PAMP) complex to promote the formation of the C1 complex, which initiates the complement cleavage cascade.
Therapeutic antibodies, such as those directed against CD20 (Rituxan), CD38 (Darzalex), and CD52 (Campath) for hematological malignancies, take advantage of this ability to generate the antibody/antigen/MAC complex to promote CDC of tumor cells and recruit macrophages to opsonize tumor cells. Thus, along with the antibody’s ability to block key signaling pathways for survival, therapeutic antibodies can promote two anti-tumorigenic effector mechanisms, CDC and opsonization, through a single complement pathway.
New roles for complement and exploiting them for new types of anti-cancer therapies
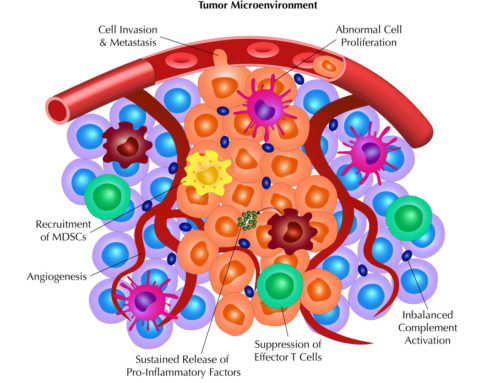
In addition to these pathways that lead to the formation of the MAC, recent studies have shown complement can have a wide range of roles besides target cell cytotoxicity and clearance. Basic immunological processes, such as antigen presentation and B and T cell responses, can be profoundly influenced by complement. In one study, the authors show that APCs express a variety of complement proteins and receptors, and that loss of complement C3 is associated with decreased antigen presentation capabilities. In another set of studies, the loss of C3aR or stimulation of C5aR1 decreases survival of CD4 T cells and promotes the differentiation of immunosuppressive CD4 T cells. In both cases, manipulation of the complement system reduces T cell responses.
To highlight those observations, complement has also been exploited in cancer vaccines. Like C3, the C5a complement can also potentiate antigen presentation by dendritic cells (DCs). To take advantage of this, a C5a-derived agonist for C5aR1 was fused to a peptide-based cancer vaccine and tested in a preclinical model of melanoma, which has shown promise. Similarly, the opsonin C3dg has been used as an adjuvant to reduce B cell activation thresholds while C3-dervied complement fragments have been used to promote memory responses by increasing antigen retention on follicular DCs. Thus, these studies further demonstrate how the power of complement can be exploited to promote anti-tumor effects.
Complement and Radiotherapy
Similarly, complement has been shown to have a role in radiotherapy, in which activation of complement is observed in response to radiotherapy-mediated cell death. Specifically, C3a and C5a are upregulated to promote antigen presentation and CD8 T cell infiltration into tumors upon radiotherapy.
Together, these studies demonstrate wide ranging roles for complement that span from cytotoxicity to antigen presentation to inflammation. More so, these studies demonstrate how the power of complement can be exploited to promote anti-tumor effects.
In our next blog, we will finish summarizing Reis et al and discuss how dysregulation of the complement system can promote tumorigenesis, the opposite effect. In addition, we’ll highlight some of the clinical aspects with regards to targeting complement.