In our previous blog, we discussed the two-predominant types of T cells, and some in vitro assays these cells are employed in to test the function and specificity of bi-specific antibodies. In this blog, we discuss the in vivo assays that test the efficacy of these antibodies and tie it all together by reviewing some data generated with these assays.
What are some T cell-based in vivo assays used to examine bi-specific antibody efficacy?
Most preclinical in vivo experiments that examine the efficacy of bi-specific antibodies are performed using immuno-deficient mice, such as the NOD scid gamma (NSG) mice. These mice are on the NOD genetic background which reduces the role of the innate immune system, including the function of macrophages and dendritic cells. Additionally, to block the development of T and B cells, these mice carry the scid mutation, which prevents V(D)J recombination, to inhibit T and B cell maturation. Finally, the IL-2 receptor gamma chain is knocked out to prevent the development of NK cells in these mice. Together, these NSG mice are immuno-deficient and are ideal for performing studies on potential therapeutics that utilize the immune system.
The NSG mice are ideal for studying molecules that utilize the immune system because the immune system of these mice can be reconstituted by introducing human T cells, or PBMCs, from different individuals through intravenous injection. Once introduced, these cells begin to circulate within the animal and can act as a surrogate immune system that is able to respond to cancerous cells or infections. Additionally, because the transferred cells are human, the cancer or infection model will be more indicative of a human response, as opposed to a mouse one. Therefore, NSG mice represent an ideal mouse model to study the human immune response.
Because the studies require functional immune cells, high quality and fully characterized pan T cells, enriched CD4, or enriched CD8+T cells obtained from reliable sources, such as iQ Biosciences, are very important for performing meaningful and reproducible experiments. If the T cells are of low quality, the cells may be non-functional and lead to erroneous data, including the interpretation of the test article as non-efficacious. Therefore, the source and quality of T cells are quite important. Most groups that use these T cells can also perform in vitro assays with the same donor prior to moving to the in vivo studies to fully bridge the data sets.
Typically, in vivo experiments are performed by injecting cells (usually, a cell line) that model a disease of interest into NSG mice. This is then followed by injection of immune cells and dosing with the test article, such as a bi-specific antibody, into the mice at different intervals. The difference in most experiments center on the readout of the efficacy of the molecule. Traditionally, the readout of tumor size is measured using calipers. However, more recent experiments monitor tumor size by using target cells that express a fluorescent or luminescent reporter and a compatible bio-imaging technology to visualize the tumors non-invasively. These imaging methods tend to be more high-throughput as multiple animals can be imaged simultaneously and require minimal handling of them. More importantly, the bio-imaging techniques can provide a more precise measurement of the tumor size compared to two-dimensional measurement using calipers. Additionally, test articles can be labeled and tracked for tumor penetration or bio-distribution.
Selection of the right in-vivo group to work with is critical to obtain reliable, robust, and reproducible results. These projects can be expensive over the long haul when considering the many types of studies that could potentially be conducted, including pharmacokinetics, distribution, efficacy, and exploratory safety experiments.
A review of data from in vitro and in vivo assays to support the development of a bi-specific antibody program
Recently, TeneoBio, a biotechnology group that develops bi-specific antibodies with their own proprietary technology, presented data using many of the assays described in this blog series. Here, we review the data for their CD3/BCMA bi-specific antibody program to illustrate how these assays are employed for development of therapeutics.
TeneoBio uses a proprietary transgenic rat platform and high-throughput sequence-based discovery engine for their antibody discovery programs, including one that centers on anti-CD3 bi-specific antibodies. Through their process, they are able to screen for antibodies with maximum epitope diversity and rapidly identify lead molecules with optimal functional capabilities.
During the development of their CD3/BCMA bi-specific antibody program, TeneoBio identified 12 lead candidates. Using an in vitro cytotoxicity assay that co-cultured T cells with BCMA expressing U266 cells in the presence of their lead candidates, they were able to identify two molecules that showed cytotoxic function which warranted further investigation (Figure 1, lead candidates boxed in red). Based on the data, one molecule (F1F) selected showed maximal killing with a low EC50, while the other selected molecule (F2B) showed maximal killing with much higher EC50. These two antibodies represent molecules that bind with different affinities while retaining maximal killing ability that were identified using an in vitro cytotoxicity assay.
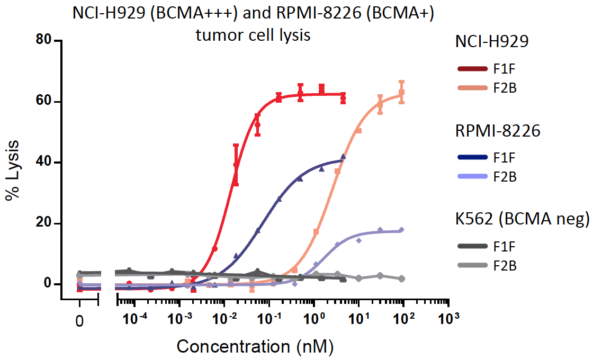
Figure 2. In vitro cytotoxicity assays of BCMA high expressing (NCI-H929) or low expressing (RPMI-8226) target cells by human pan-T cells in the presence of TeneoBio’s lead CD3/BCMA bi-specific antibodies. K562 target cells that do not express BCMA were used as a negative control.
F1F and F2B underwent further characterization using the same in vitro cytotoxicity assay. However, in this experiment, three target cell lines with different expression levels of BCMA were used. As shown in Figure 2, the highest expressing line, NCI-H929, was most susceptible to lysis, while the lower expressing line, RPMI-8226, was less susceptible. As a control, K562 cells, which do not express BCMA, were not lysed. This experiment corroborated the initial observation that the two chosen molecules could indeed kill BCMA-expressing target cells and that cytotoxicity was specific and proportional to expression level.
To further demonstrate that these two candidate antibodies were specific and elicited an immune response, cytokine release was interrogated. The supernatant from the co-culture of the cytotoxicity assays were collected and examined for cytokines using a cytokine bead array, which tests for a number of cytokines simultaneously. As shown in Figure 3, IL-2 and IFN-g was detected in the supernatants of cultures that included BCMA expressing lines, T cells, and F1F or F2B. In contrast, K562, the BCMA non-expressing line, did not promote cytokine production. To further demonstrate specificity, versions of F1F and F2B that did not have the arm that bound BCMA did not elicit IL-2 or IFN-g. Thus, this in vitro cytokine release assay further showed the specificity and functional ability of these molecules.
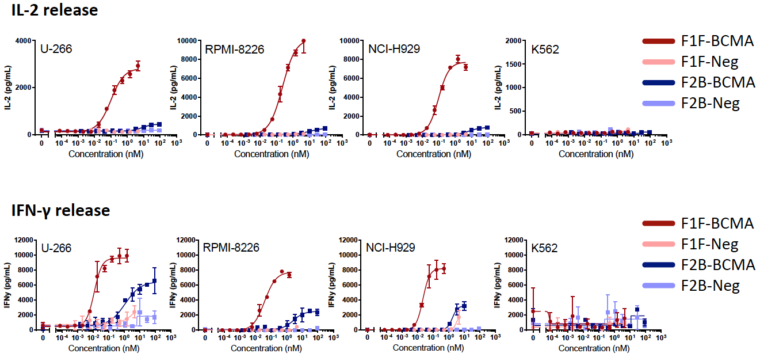
Figure 3. In vitro cytokine release assay of supernatant from cultures with TeneoBio’s lead CD3/BCMA bi-specific antibodies, target cells, and pan T cells. IL-2 and IFN-g were measured simultaneously using a cytokine bead array kit. Target cells with varying expression levels of BCMA were used.
Finally, Figure 4 shows images from an in vivo assay in which NSG mice were injected with a BCMA expressing cell line (RPMI-8226) and PBMCs, and then dosed with their two lead candidates. In this experiment, RPMI-8226 expressed a reporter molecule to measure tumor size using a bio-imaging instrument. Compared to the vehicle control, mice treated with either antibody demonstrated protection from tumor engraftment and growth as evidenced by less detection of the reporter molecule expressed by the cell line.
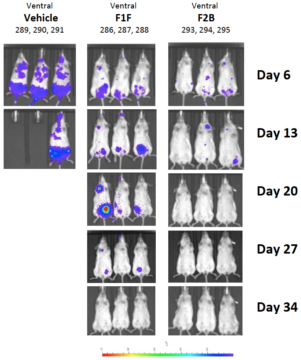
Figure 4. In vivo assay to test efficacy of TeneoBio’s lead CD3/BCMA bi-specific antibodies. Briefly, mice were injected with RPMI-8226 target cells that express a reporter and pan T cells, followed by dosing with TeneoBio’s lead CD3/BCMA bi-specific antibodies. Mice were imaged at various timepoints for tumor size.
As shown using TeneoBio’s CD3/BCMA bi-specific antibody platform, in vitro and in vivo assays complement each other nicely to demonstrate specificity, function, and efficacy to identify and develop lead compounds. It is interesting to note that TeneoBio’s lead bispecific molecule is very different from other BCMA bi-specific programs in that TeneoBio’s molecule contains two anti-BCMA heavy chain variable domains linked to a unique anti-CD3 T-cell recruiting arm, which maximizes killing of multiple myeloma target cells with markedly reduced cytokine release. This bodes well for future patients as there should be superior efficacy with significantly lower cytokine release that can accompany these types of redirected T-cell therapies.
Overall, these assays provide powerful information and insight for any researcher who is searching to further develop their therapeutic programs. Contact iQ Biosciences to find out ways we can help you with these in vitro assays and in vivo assays, as well as the preclinical development of your program.