- Evaluate your immune checkpoint (CTLA-4, PD-1, TIM-3, LAG-3, TIGIT, OX40, GITR, etc.) biologic or small molecules in functional assays
- Assays to measure activity (CellTiter-Glo, LDH, etc.) can be performed with appropriate cell lines that express your biologically relevant target in presence of your biologic or molecules
- Assays to measure proliferation, expression of cell surface activation markers, and cytokine expression can be performed with primary cells using non-antigen specific (superantigens) or antigen specific approaches in the presence of your molecule
- Personalized experimental design to ensure the most relevant data is obtained
- All assays are performed with high-quality reagents and strict controls by experienced immunologists
Service Overview
About Immuno-oncology and Immune Checkpoint Proteins
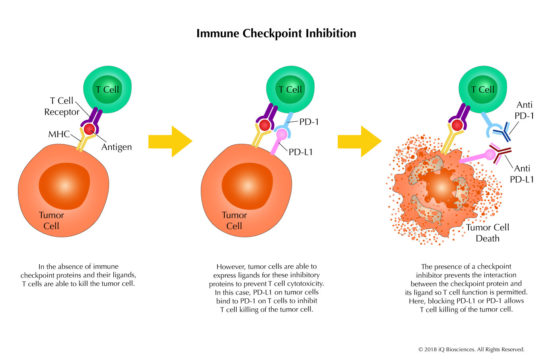
Over the last 10 years, there has been a tremendous amount of research in the field of immuno-oncology, which aims to study the ability of an individual’s immune system to attack and destroy tumor cells. Through these studies, cell surface molecules called immune checkpoint proteins were discovered, which function to down-regulate the immune response by inactivating lymphocytes. On the heel of these findings, therapeutics called checkpoint inhibitors were developed that modulated the immune system to sustain and/or promote lymphocyte activation to destroy cancer cells.
Activation of lymphocytes is exquisitely controlled to ensure an adequate immune response is elicited to clear foreign pathogens. However, the response must also recede afterwards to prevent unwanted attacking of host tissue. To this end, lymphocytes express proteins called immune checkpoint proteins that engage their cognate ligands on host cells to down-regulate activation and the immune response. Thus, the immune response is carefully regulated to optimally respond to pathogens, but also tempered upon resolution.
Besides clearing foreign pathogens, lymphocytes also surveil the body looking for aberrant or diseased cells, destroying them before they can become cancerous in a process called immuno-surveillance. However, cancer cells have developed mechanisms to evade this surveillance; one of which is to express ligands for checkpoint proteins to prevent and/or weaken lymphocyte activation and response.
It has been thought that the power to clear infected and aberrant cells by lymphocytes can be harnessed to treat cancer. After years of research, the first immune checkpoint inhibitor, which targeted CTLA-4, was approved by the FDA in 2011; providing proof of concept that checkpoint inhibitors can be used to modulate the immune system to attack cancer cells. Mechanistically, the CTLA-4 therapeutic antibody works by blocking the interaction of CTLA-4 on cytotoxic T lymphocytes (CTLs) with its ligand, B7, to prevent inactivation of the CTLs. In this scenario, the CTLs are activated or remain activated to search and destroy cancer cells. Following the approval of this first immune checkpoint inhibitor, many biotechnology and pharmaceutical companies began to search for other targets and develop molecules to target them. Today, the industry is diligently developing immune checkpoint inhibitors that target checkpoint proteins to treat cancer.
iQ’s Biosciences’ Immune Checkpoint Assays
At iQ Biosciences, we are an immunology CRO with years of experience helping companies develop their immune checkpoint inhibitors, whether they are antibodies, peptides, or small molecules, using our immuno-oncology assays. We can help design a roadmap that begins with binding assays to screen for good candidates, proceeds to interaction assays with selected candidates, then progresses finally to functional assays that test for immuno-modulatory function. With this roadmap, we can demonstrate the binding and functional properties of your molecule, along with its potential mechanism of action.
Discover Your Molecules’ Potential to Modulate Immune Function with iQ’s Immune Checkpoint Assays
As part of that roadmap, checkpoint inhibitors have to be tested for immuno-modulatory activities using functional assays, which iQ Biosciences has extensive expertise with. See our Immune Checkpoint Binding and Interaction Assays for initial characterization of your immune checkpoint inhibitors. In most experiments we perform, we begin by stimulating T cells in the presence of the checkpoint inhibitor using a variety of activating reagents. We can stimulate cells using various antigen-specific or non-antigen specific activation methods, including SEB or anti-CD3/CD28 antibodies. Following stimulation, execution of at least one of the following functional assays is performed: T cell proliferation assay or cytokine production assay. For the T cell proliferation assay, propagation of T cells is measured by CFSE dilution or Cell Titer Glo following the stimulation period. The supernatant can simultaneously be collected from the T cell proliferation cultures to assess cytokine production using Cytokine Bead Arrays or Meso Scale Discovery technology in our cytokine production assays. Additionally, these functional assays are all offered in a high throughput method so multiple molecules and conditions can be incorporated. See Figure 1 for an example of our immune checkpoint functional assay using cytokine production (IL-2) as a readout.
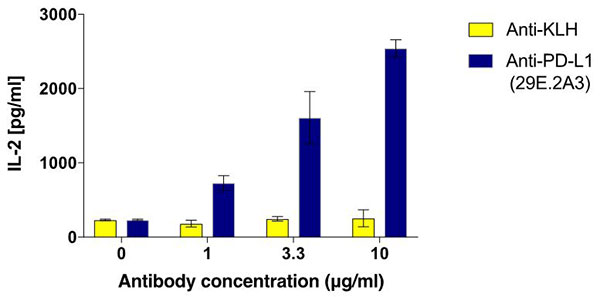
Figure 1. Enhancement of IL-2 production with addition of PD-L1 antibody using antigen-specific stimulation system. Tetanus toxin-specific PBMCs were stimulated with tetanus toxin in the absence or presence of PD-L1 antibody (clone 29E.2A3) for 5 days. At the end of the stimulation, the supernatant was collected and assessed for IL-2.
iQ can also perform a unique immune checkpoint functional assay that uses a special reporter cell line to determine if your inhibitor can restore immune cell function. Here, the reporter cell line expresses the checkpoint protein, while the target cell expresses its ligand. Blocking their interaction leads to activation of the reporter cell line and expression of luciferase. A substrate is then added to the culture to measure luciferase expression. This is an extremely reliable, high-throughput method to screen a large set of molecules for blocking activity.
iQ Biosciences – Your Immunology CRO for Checkpoint Inhibitor Development
Together, iQ is your immuno-oncology CRO that can help design a roadmap to develop your checkpoint inhibitors, as well as execute studies to bring that roadmap into focus. Please contact us for more information.