- Help predict the potential magnitude of complement-mediated target lysis conferred by your therapeutic antibody with iQ’s complement-dependent cytotoxicity (CDC) assay
- Personalized experimental design ensures the most relevant CDC data are generated with your molecules
- All assays are performed with high-quality reagents and strict controls by experienced immunologists
- Data is provided in appropriate formats, including PowerPoint slides and/or direct data files
Service Overview
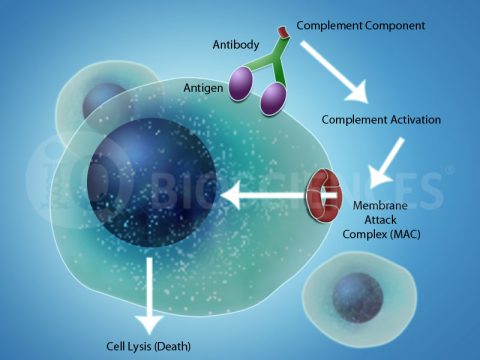
About Complement-dependent Cytotoxicity (CDC)
Complement-dependent cytotoxicity (CDC) is one mechanism by which antibodies can mediate specific target cell lysis through activation of an organism’s complement system. The complement system consists of over 20 small proteins synthesized by various tissues and cell types, including immune cells, and is regulated by 3 different pathways. After activation of the pathway, complement components in the serum are recruited to target cells that are opsonized by antibodies. Opsonization is the binding of an antibody to its cognate antigen on the cell surface. Once bound to the target cell, the complement components undergo multiple enzyme activation and cleavage events that lead to lysis of the antibody-bound target cell. Additionally, activation of the complement system leads to inflammation and subsequent recruitment and stimulation of immune cells.
CDC plays a role in general human health by eliminating foreign pathogens, such as bacteria and extracellular organisms, and aberrant cells that would otherwise promote illness. In addition, CDC is a mechanism by which therapeutic antibodies for cancers, such as those that target CD20 and CD38, may mediate their effect. Therefore, during drug development, potential therapeutic antibody candidates will be screened for CDC activity to find ones that will have these potent anti-cancer effects.
Factors Important for CDC Activity
iQ has extensive experience with CDC assays and can help you design the best experiment based on key factors that can influence CDC. Four well-studied factors that can enhance or decrease CDC are:
- Antibody isotype – Certain antibody isotypes have better CDC abilities than others. In general, IgG3 > IgG1 >>> IgG2 ≈ IgG4 in terms of CDC capability. Additionally, engineering specific amino acid substitutions into either the hinge region or CH2 domain of the antibody can improve CDC activity.
- Expression level of antigen on target cells – The target cell line should express sufficient target antigen for the antibodies to bind and recruit complement. Without appropriate amounts of antigen, complement activation cannot be initiated.
- Expression of complement regulators on target cells – The target cell line may express surface proteins that inhibit or regulate complement activation. Some of these membrane bound regulators include CD46, CD55, and CD59.
- Complement source – Complement is found in the serum and is added to the assays to promote CDC. Therefore, it is important to know the organismal source of the serum to ensure that it is compatible with the target cells since some cells of a certain origin are not compatible with serum from certain animals.
At iQ, we have various methods to assay for cell death. We can perform CDC assays by flow cytometry wherein cells are stained with a DNA intercalating dye to identify dead (membrane-permeable) target cells. In these experiments, cells are cultured with antibody and serum, followed by staining with the dye and analysis on the flow cytometer. Our high-throughput flow cytometer allows us to test many conditions and broad dose responses in a single assay. This type of assay provides more sensitivity as it directly interrogates cell death.
At iQ, we have refined protocols with quality-controlled complement sources, including human, non-rodent, and rodent, that can be used for product characterization or therapeutic candidate screening purposes as described in ICH guideline Q6B – Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products (2.1.2 Biological Activity). In addition, with our extensive knowledge of factors that influence CDC, iQ can help design the right experiments for you to determine the CDC potential of your therapeutic antibodies.
Our goal is to provide the most robust and consistent approaches to obtain meaningful results for you. Together, we will design assays and analyze data to functionally characterize your antibody for bioactivity and/or guide preclinical transition from in-vitro to in-vivo studies.
Request a CDC Fact Sheet
Interested in learning more about our CDC Bioservices? Complete the form below to request a Fact Sheet.
