- High-quality rhesus macaque PBMCs sourced responsibly from a vetted third party facility
- High viability (> 90%) after thawing
- Used for a wide variety of safety assessment and functional assays
- All orders come with an iQ Certificate of Analysis
- Normally ships out same business day
Rhesus Monkey Peripheral Blood Mononuclear Cells (PBMCs)
$325.00 – $510.00
- Description
- Additional information
- Donor Request
- Ethics
- iQ Experience
- Shipping Information
- Distributors
Description
About the Rhesus Monkey (Rhesus Macaque)
Rhesus macaques are one of the most commonly utilized non-human primates in biomedical research. They are employed in numerous research areas, such as immunology, neuroscience, oncology, infectious disease, and toxicology due to their physiology.
Application Summary for Rhesus Monkey PBMCs
Rhesus monkey PBMCs can be used for a wide variety of safety assessment and functional studies. They are commonly employed in pre-clinical settings to ensure biologics are not eliciting unwanted functions, such as cytokine release and toxicity. They are also used in many immunology-based applications, such as ELISPOT, cytokine production assays, and cytotoxicity assays to measure ADCC or CDC in response to biologics. In addition, rhesus monkey PBMCs can also be used in ex-vivo applications for cell population characterization.
Isolation of PBMCs
Peripheral blood is obtained from responsible facilities that have many years of experience collecting samples. Since the facilities are local, the blood is quickly transported to iQ Bioscience’s laboratory for processing.
Once at our lab, PBMCs are isolated by performing density centrifugation with Ficoll, a high molecular weight sucrose solution, to remove red blood cells under sterile conditions. Depending on the species (human or non-human) of blood, either 1.077 or 1.084 g/ml Ficoll is used, resulting in a perfect layer of mononuclear cells that are separated from plasma, platelets, granulocytes, and erythrocytes. Additional quality control steps are taken to isolate and prepare PBMCs that ensure the highest viability for cryopreservation and downstream experimental applications.
Cryopreservation and Storage
Our rhesus PBMCs are cryopreserved carefully using iQ Biosciences’ cryopreservation protocol that ensures high viability (> 90%) after thawing.
Cells should be stored at < -120°C once they are received, such as within a liquid nitrogen tank (vapor phase).
Quality Control Process
All of our non-human primate donors have been tested for Herpes B virus, Simian Retrovirus (SRV), and tuberculosis. Additionally, we implement random sampling per lot to test for viability and cell counts to ensure they meet specifications, which are recorded on the Certificate of Analysis that is included with each shipment. Cell counts are obtained using a manual hemocytometer and then cross-referenced with an automated cell counter. Each lot is also characterized for unique cell populations by immunophenotyping, in which the results are also recorded on the Certificate of Analysis.
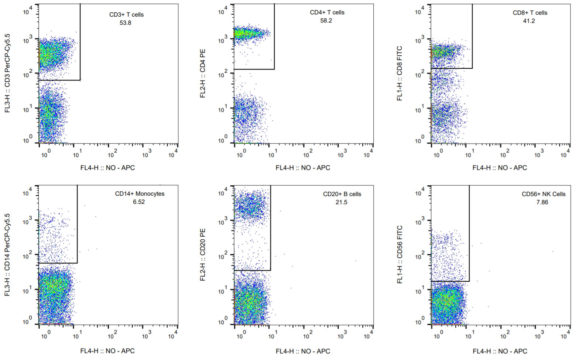
Figure 1. Flow profiles of T cells (CD3, CD4 gated on CD3+ population, and CD8 gated on CD3+ population), monocyte (CD14), B cell (CD20), and NK cell (CD56) populations in rhesus PBMCs. The specific immune cell subset and its percentage is listed on the top right of the flow profile. Briefly, rhesus PBMCs were stained with antibodies for CD3, CD4, CD8, CD14, CD20, and CD56, followed by flow cytometric analysis.
Additional information
| Available Size(s) | |
|---|---|
| Format | |
| Cell Type | |
| Tissue Type | |
| Species | |
| Viability | > 90% |
We are happy to accommodate the following donor requests if inventory is available. Feel free to inquire prior to ordering, or specify your interest in the "Order notes" section during checkout, and we will follow-up with you shortly after your order is processed, if necessary:
- Single or multiple donors for a particular product (SKU). Indicate the number of vials from each individual donor
- Donor(s) with a higher percentage of the cells of interest (e.g., B cells, CD4+ cells, CD8+ cells, NK cells, etc.)
We are Committed to Ethical Practices
iQ Biosciences’ primary cell products from non-human primates are collected under approved IACUC protocol, which was developed in consultation with the Attending Veterinarian and is consistent with current veterinary standards. The collection facility is AAALACi and PHS accredited, and all animal housing, handling, and research protocols are consistent with standards set forth by the Animal Welfare Act, NIH's Policy on Humane Care and Use of Animals and the Guide for the Care and Use of Animals. All animal housing and handling is done in such a way to minimize stress and risk of injury to the animal.
For US customers, we ship via FedEx Overnight Shipping. Shipping charges will vary per shipping address (based on ZIP code) and are estimated to be $140.
For international (non-US) customers, we work closely with you and our couriers to ensure all necessary documentation is in place for international shipments to significantly reduce the chance of delays at Customs. For the export of non-human primate samples, this includes preparing CITES permits, as well as any other documentation as required by country. Please submit an inquiry to orders@iqbiosciences.com for your estimated time of delivery and shipping charges.
Austria
Hölzel Diagnostika Handels GmbH
Tel: +49 221 126 02 66
Email: info@hoelzel.de
Web: https://www.hoelzel-biotech.com/
Canada
Cedarlane
Tel: +1 (289) 288-0001
Toll Free (North America): +1 (800) 268-5058
Fax: +1 (289) 288-0020
Email: sales@cedarlanelabs.com
Web: https://www.cedarlanelabs.com
China
BIOHUB INTERNATIONAL TRADE CO., LTD.
上海起发实验试剂有限公司
Address: Chuansha Rd #6619, Pudong, Shanghai, Zipcode: 201200 P.R.China
Tel: 0086-021-50724187
Phone: +86-15921799099
Fax: 0086-021-50724961
Email: sale3@78bio.com
Web: www.qfbio.com
European Union
Caltag Medsystems Ltd.
Email: office@caltagmedsystems.co.uk
Web: https://www.caltagmedsystems.co.uk
tebu-bio
Web: https://www.tebu-bio.com
Or Find a local contact
Germany
Hölzel Diagnostika Handels GmbH
Tel: +49 221 126 02 66
Email: info@hoelzel.de
Web: https://www.hoelzel-biotech.com/
Zageno
Web: https://zageno.com/
Ireland
2B Scientific Ltd
Tel: +44(0) 1869 238033
Fax: +44(0) 1869 238034
Email: sales@2BScientific.com
Web: https://www.2bscientific.com
India
Cell & Gene BioSolutions Pvt. Ltd.
#478 C, SLV Complex, Raghavendra Swamy Mutt Road
Opp. Turahalli Water Tank, Turahalli, Subramanyapura Post
Uttarahalli Hobli, Bengaluru-560061, Karnataka, India
Phone: +91 97317 14670
Phone: +91 98809 25033
Email: info@cgbios.com
Web: www.cgbios.com
Japan
Cosmo Bio Co., Ltd.
Tel: +81 (03) 5632 9610
Fax: +81 (03) 5632 9619
Email: nsmail@cosmobio.co.jp
Web: https://www.cosmobio.co.jp
Qatar
Sedeer Medical Services and Trading LLC
Tel: +974 4434 9191
Email: info@sedeer.com
Web: https://sedeer.com/
Singapore
Omnicell Pte Ltd
Tel: +65 6747 0201
Email: enquiry@omnicell.com.sg
Web: https://omnicell.com.sg/</a
South Korea
BioClone
Tel: +82-2-2690-0058
Email: bioclone@bioclone.co.kr
Web: http://www.bioclone.co.kr
Switzerland
Hölzel Diagnostika Handels GmbH
Tel: +49 221 126 02 66
Email: info@hoelzel.de
Web: https://www.hoelzel-biotech.com/
Taiwan
Hycell International Co. Ltd.
Tel: +886-2-2877-1122
Fax: +886-2-2876-1520
Web: http://www.hycell.com.tw
United Kingdom
2B Scientific Ltd
Tel: +44(0) 1869 238033
Fax: +44(0) 1869 238034
Email: sales@2BScientific.com
Web: https://www.2bscientific.com
Caltag Medsystems Ltd.
Tel: +44 (0)1280 827460
Fax: +44 (0)1280 827466
Email: office@caltagmedsystems.co.uk
Web: https://www.caltagmedsystems.co.uk
tebu-bio
Tel: +44 (0)1733 421880
Fax: +44 (0)1733 421882
Email: uk@tebu-bio.com
Web: https://www.tebu-bio.com
Zageno
Web: https://zageno.com/
United States
Fisher Scientific
Tel: 1-800-766-7000
Web: https://www.fishersci.com
Quartzy
Web: https://www.quartzy.com
VWR International
Tel: 1-800-932-5000
Web: https://www.vwr.com
Zageno
Web: https://zageno.com/