- Help predict cytokine release (i.e. cytokine storms) induced by antibody-based therapeutics in first in-human trials or primate safety studies with iQ’s Cytokine Release Assay
- Assess cytokine release with immunogenicity assays in response to therapeutic molecules using appropriately designed studies based on our direct experience with FDA feedback (pre-IND meeting comments, etc.)
- Data is provided in appropriate formats, including raw data, PowerPoint slide summary, and optional formal report to supplement potential IND or EMA filings
- All assays and methods are performed according to FDA and EMA recommended guidelines
- Our high throughput system and workflow with qualified donors give you high quality results with rapid turnaround
Service Overview
Cytokine Release Assay (CRA) Background
With the current trend of developing large or small molecule-based immune-modulatory therapeutics for various oncology and autoimmune indications comes the risk of unwanted immune side effects in first in-human trials. At iQ Biosciences, we aim to provide drug developers additional information about their molecule before proceeding to clinical trials. In particular, we provide immunogenicity assays (like this Cytokine Release Assay) to screen for markers that may predict potentially dangerous side effects after infusion into humans.
One of the most dangerous side effects from administration of a therapeutic molecule is a cytokine storm, or cytokine release syndrome (CRS)1, and a common molecule responsible for these “cytokine storms” are antibody-based therapeutics. After administration of the therapeutic, the patients experience a release of pro-inflammatory cytokines, which results in symptoms that range from fevers and rashes to more severe and life threatening ones, such as cardiac arrest or organ failure1. Needless to say, these are unwanted side effects of the biologic and can be predicted with cytokine release testing.
TGN1412 Causing a Cytokine Storm
The most well documented case of a biologic that caused a cytokine storm is TGN1412, an anti-CD28 “superagonist” antibody that was proposed to treat certain autoimmune diseases and hematologic cancers2. The adverse events experienced by patients after administration included pain, amnesia, diarrhea, vomiting, fever, and severe multi-organ failure1. More importantly, the cause of these life-threatening effects was subsequently attributed to a cytokine release storm1. However, the pre-clinical safety testing showed no evidence TGN1412 would cause such severe and adverse reactions in first-in-human trials. While TGN1412 is an extreme case, approved HER2 and CD52 antibodies are other case studies which have been associated with pro-inflammatory clinical infusion reactions2. (For more information, see our blog on cytokine release assays.)
Current Regulatory Agency Recommendations Regarding Cytokine Storms
As a consequence of the lack of predictive power between pre-clinical and in-human results from the TGN1412 studies, a standard method to better predict cytokine storms was developed by the European Medicines Agency (EMA)2. The FDA also strongly recommends performing experiments to better predict cytokine storms3. At iQ Biosciences, we have developed expertise in better predicting cytokine storms by performing and optimizing cytokine bead assays (CBA) to assess the release of cytokines from human PBMCs in response to therapeutic molecules. Furthermore, cytokine detection assays and methods are in concordance with EMA and FDA recommended guidelines, including the measurement of serum IL-2, IL-6, IFN-γ, and TNF-α.
Please see our Cytokine Release Assay Workflow page describing our protocol and examples of data from our CRAs:
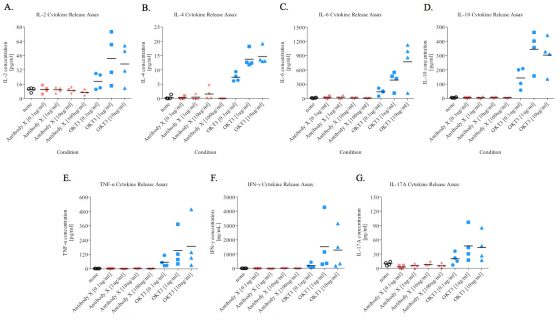
Mean serum concentration of IL-2, IL-4, IL-6, IL-10,
TNF-α, IFN-γ, and IL-17A from ‘Antibody X’ treated PBMCs.
In the competitive field of developing immune-modulatory therapeutics, or any antibody-based therapeutic for that matter, ensuring the molecule does not elicit a cytokine storm in first in-human trials is critical. In addition, with the growth of companies specializing in biosimilars, as well as new technologies, such as bispecific (BiTE) or mulitmeric antibodies, obtaining cytokine data via multiplexed cytokine quantitation is just as important in providing pre-clinical data before in-human trials. Accordingly, the financial and, more importantly, patient health setbacks can have serious implications. With iQ Biosciences’ expertise in cytokine profiling to assess the expression of a variety of cytokines, drug developers can use our predictive human cytokine data to help assess the direction of their potential therapeutics and research programs.
How can iQ Biosciences help achieve your goals the smarter way?
References:
- Suntharalingam, G. et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. The New England Journal of Medicine 355, 1018-1028 (2006).
- Stebbings, R., Eastwood, D., Poole, S. & Thorpe, R. After TGN1412: recent developments in cytokine release assays. Journal of Immunotoxicology 10, 75-82 (2013).
- Guidance for Industry Immunogenicity Assessment for Therapeutic Protein Products. FDA DRAFT GUIDANCE (Clinical/Medical, February 2013).
Request a Cytokine Release Fact Sheet
Interested in learning more about our Cytokine Release Bioservices? Complete the form below to request a Fact Sheet.
