PART 1: Why Drug Development Values TCR Studies
What are Tissue Cross-Reactivity studies?
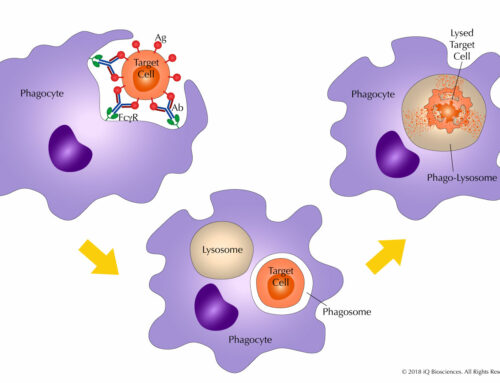
Tissue cross-reactivity (TCR) studies are screening assays used to identify the specific and non-specific binding of test biologics (i.e. antibodies or antibody-like proteins) in various human or non-human tissues. If human tissues are used, the primary goal is to identify potential off-target, cross-reactive epitopes across a wide range of tissues. If non-human tissues are used, these studies can help identify the most relevant species with a similar target expression profile to human. These studies are routinely performed by leading drug development companies like Pfizer, Genentech, and Genzyme, but can also be out-sourced to well respected contract-research organizations such Charles River Laboratories, Pantomics, and BioReliance.
Typically, TCR studies are done by Immunohistochemistry (IHC) with the test biologic on a panel of tissues from human and animal species that are considered (or selected) for toxicity evaluation. In many cases, the staining profiles of human and non-human tissues on a test biologic is different in terms of distribution and intensity, suggesting that data from TCR studies should be carefully interpreted and in the context of preclinical studies on a case-by-case basis.
Why are TCR studies important?
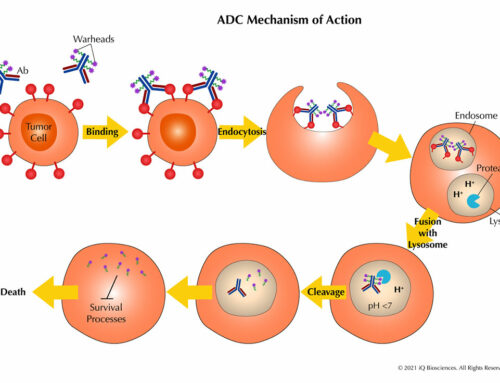
TCR studies are important because they are used to support in-human clinical trials when the initial Investigational New Drug (IND) Application / Clinical Trial Application (CTA) is filed with regulatory agencies for a test biologic. These studies are an essential part of the preclinical safety assessment package. The studies should be performed with the “real” biologic, such as GMP-level material that would be used in the clinic, rather than a parental and/or unmodified form, on a panel of tissues from three different unrelated human donors and animals prior to in-human testing. However, some preclinical groups prefer to use research level material for IHC method development and for a non-GLP survey to get a possible preview. This has the benefit of accelerating timelines and having the option to not report findings since it is performed at a research level.
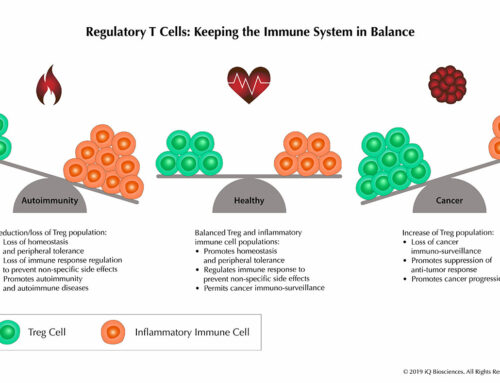
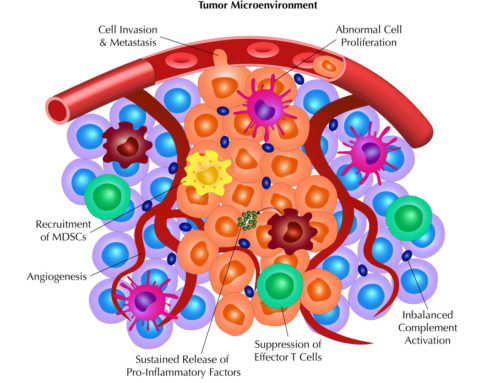
Scientifically, the staining pattern and distribution of staining on the panel of human tissues helps alert investigators to potential toxicity toward certain organs or has the possibility to expand the potential indications pending the identification of sites of on-target binding that were not previously identified. Since safety assessment is the primary goal, the staining intensity can also lend insight into potential toxicity. In conjunction with data from animal tissue, TCR studies can also assist in determining the right species to perform in-vivo toxicity studies as well as in-vivo pharmacodynamic modeling studies to obtain useful quantitative information on the effect of a cross-reacting biologic.
Value of TCR studies in industry
Bussiere et al (2010 Regulatory Toxicology and Pharmacology) conducted a survey on pharmaceutical and biotechnology companies regarding the use and value of IHC-based TCR studies. In the study, they found that observations from the TCR studies did not influence development strategy in 95% of the biologic targets surveyed. 73% of the targets did not use TCR studies as the only determinant for selecting species for toxicity studies. Further, only 2% of the molecules used TCR studies as the sole data for determining potential risk in patients. Together, these findings further illustrate that data from IHC-based TCR studies are best used in conjunction with data from other types of study for evaluation of test biologics.
Stay tuned for the next part of this series: PART 2: What to Consider & How to Prepare for TCR studies